ICE® is a fully validated software compliant with industry regulations and guidelines (GxP-GCP, GAMP 5, FDA CFR 21 part 11 , EU Annex 11, MDR, GDPR, HIPAA) already used and validated by healthcare professionals in CROs, research organizations, pharmaceutical industries, scientific foundations, and hospitals in Italy and around the world.
Build your studies and significantly simplify the data collection process at reduced costs for clinical trials.
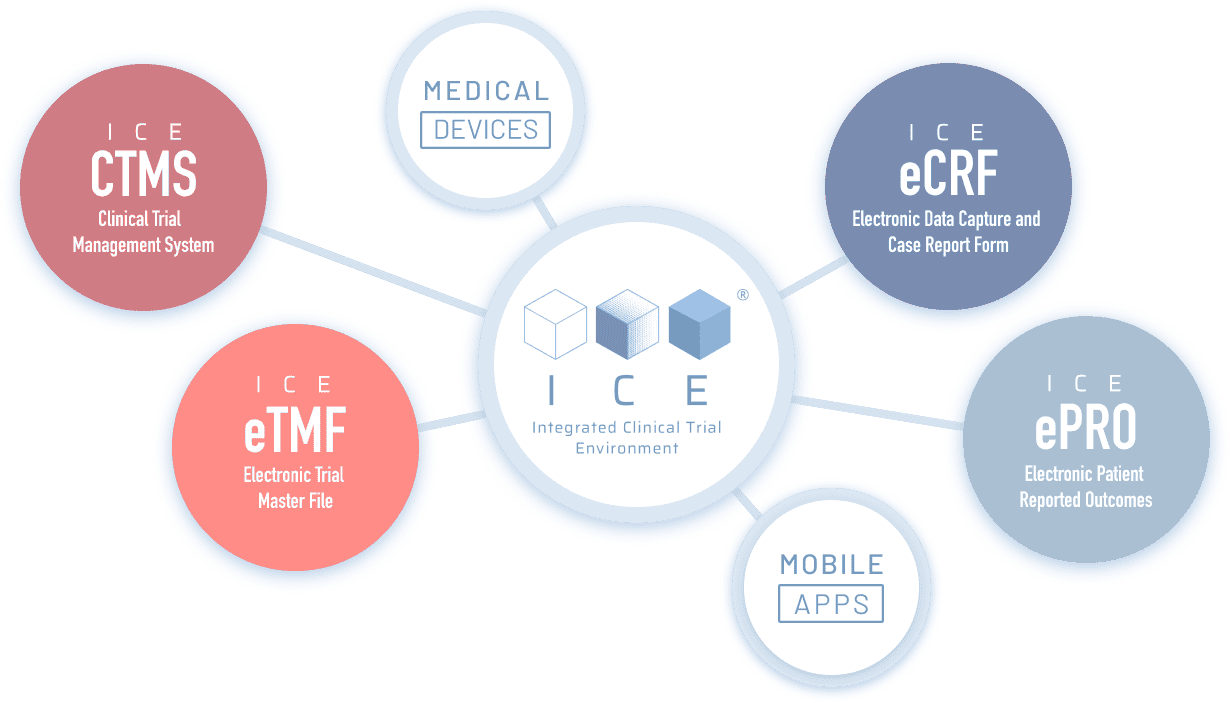
One ecosystem, multiple applications
Thanks to the use of API communications, ICE® can communicate with wearables and other apps to monitor vital signs, detect and record events.
It also integrates with other EDC systems and laboratory systems (LIMS).
ICE® Ecosystem is composed of the following products:
What they say about us
Effectiveness of a telemonitoring intensive strategy in the early rheumatoid arthritis: comparison with the conventional management approach. Salaffiet al. BMC Musculoskelet Disorders
Intravesical administration of combined hyaluronic acid (HA) and chondroitin sulfate (CS) for the treatment of female recurrent urinary tract infections: a European multicentre nested case-control study. Ciani et al. BMJ Open
Lung Cancer App (LuCApp) study protocol: a randomised controlled trial to evaluate a mobile supportive care app for patients with metastatic lung cancer. Ciani et al. BMJ Open
Cangrelor, Tirofiban and Chewed or Standard Prasugrel Regimens in Patients with ST-Segment Elevation
Myocardial Infarction: Primary Results of the FABOLUS FASTER Trial. Gargiulo et al. Circulation
2020; 142: 441-454
Preparation of ICE® for CDASH nomenclature developments and overall system performance optimization.
New section for drug management and optimization of existing features.
We are excited to announce the launch of ICE® ePRO, the innovative solution designed to transform clinical trial management by
The new version of ICE® eCRF introduces a dedicated section that allows users to select and export questionnaires to be
The latest update introduces several new features that improve study management, enhance security, and optimize data export. One of the
ICE is developed by: