The proprietary applications related to the platform ecosystem are:
- ICE® eCRF: Electronic Case Report Form
- ICE® ePRO: Electronic Patient Reported Outcome
- ICE® eTMF: Electronic Trial Master File
- ICE® CTMS: Clinical Trial Management System
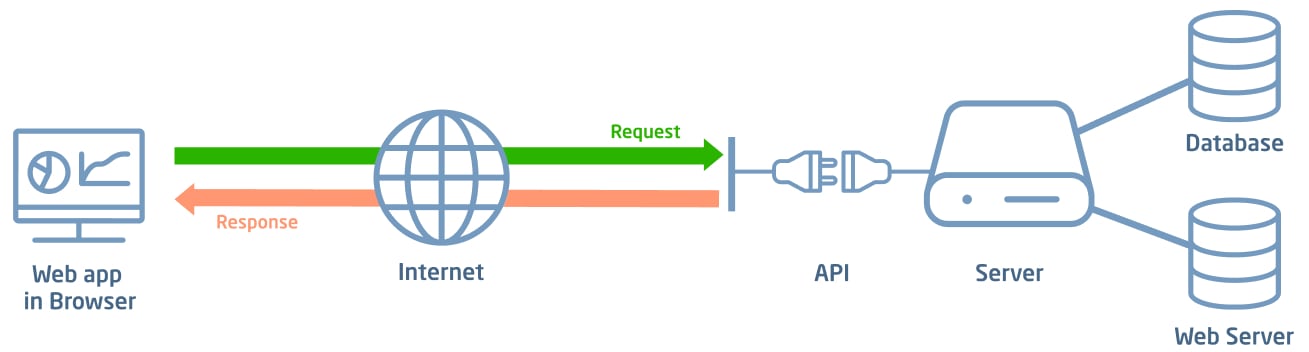
All solutions feature a user-friendly web-based interface and are distributed as SaaS (Software as a Service) applications. Users only need a browser on their device to interact with the platform. Each application is developed with a multi-tenant architecture, enabling centralized and shared software management that optimizes resources, updates, and maintenance for every client. Development and maintenance adopt the latest technologies to ensure security standards (e.g., OWASP Top 10).
Key Validation Activities
Throughout the entire lifecycle of the system, we perform rigorous Computer System Validation (CSV/CSA) activities to ensure our products meet the highest standards of quality and compliance with applicable regulations, including (FDA 21 CFR Part 11, Eudralex Vol. 4 Annex 11 and related guidelines, GDPR, HIPAA).
These activities include:
- Validation Planning: We define a detailed plan outlining the objectives, resources, and timelines for system validation.
- Risk Analysis: We identify and assess potential risks associated with the system to ensure they are properly mitigated.
- Validation Testing: We conduct thorough testing to verify that the system is securely installed and functions correctly under all expected operating conditions.
- Detailed Documentation: We maintain complete and accurate documentation of all validation activities, including test results and any corrective actions. This ensures transparency and allows auditors to examine every aspect of the validation process.
- Review and Approval: Validation results are reviewed and approved by a team of experts to ensure compliance with regulatory requirements and corporate standards.
- System Updates: Every change or enhancement to the system or its configurations is tracked and authorized through a change management mechanism. Updates are executed and validated in a test environment before release.
ICE Platform Development Timeline
OCTOBER
ICE® eTMF 3.11.0 - Introduction of study visiblity and center specific for all users.
JULY
ICE® eCRF 3.11.0 - ICE® readiness for developments in CDASH nomenclature and optimization of overall system performance.
JUNE
ICE® eCRF 3.10.0- New section for drug management and optimization of existing functionality.
MAY
Introducing electronic Patient Reported Outcome, ICE® ePRO 1.0.0 – the innovative, validated web-based system that enables patients to directly complete electronic questionnaires, with the goal of improving both care and reporting. Thanks to ePRO, the collection of digital health data becomes decentralized, efficient, and reliable.
MAY
ICE® eCRF 3.9.0 - ePRO module, improvements to audit and eQuery functionality.
JANUARY
ICE® eTMF 1.1.0 - The workflow for study closure and approval has been implemented, along with a new feature that enables document export based on user-defined filters.
DECEMBER
ICE® eCRF 3.8.0 - Protocol deviation: new section to track open queries in this way.
SEPTEMBER
ICE® eCRF 3.7.0- IDE/Editor: Added a new component called apibutton, visible only through permission settings. Administration tools: Introduced a new button to enable visibility permissions for the apibutton component.
SEPTEMBER
Introducing electronic Trial Master File, ICE® eTMF 1.0.0 – the digital system used to manage and archive the essential documents of a clinical study. It replaces the traditional paper-based Trial Master File (TMF), which contains the documentation required for conducting clinical trials.
MAY
ICE® eCRF 3.6.0- PDF printing optimization; added drug coding functionality; added “Associate all sites” option for users.
FEBRUARY
ICE® eCRF 3.5.0 - SAS Export: added new functionality for exporting data in SAS format.
NOVEMBER
ICE® eCRF 3.4.0 - Helpdesk: Ticket creation now available directly from ICE3. Event mail: Improved handling of “component value” events.
OCTOBER
ICE® eCRF 3.3.0- Integration with LimeSurvey for clinical data.
SEPTEMBER
ICE® eCRF 3.2.0- URV Grid: Added a new section to better visualize all URVs completed by a patient. Clinical data import: Introduced a system for bulk importing clinical data.
JULY
ICE® eCRF 3.1.0 - Audit trail: Now subject only to soft-delete; eCRF file tracking: Added logging of downloaded uploaded eCRF files.
MARCH
ICE® eCRF 3.0.0 Release - New Infrastructure of multitenancy.
Nasce Clinical Trial Management System, ICE® CTMS 1.0 - che consente anche grazie al dialogo con le eCRF create dalla piattaforma ICE, di ottenere in tempo reale i dati necessari per monitorare il raggiungimento dei KPI delle sperimentazioni cliniche
ICE® eCRF 2.0.0 - Go-live of the first clinical trials.
Introducing Integrated Clinical Trial Environment, ICE® eCRF 1.0 – the EDC platform for collecting and monitoring clinical data, an essential tool supporting clinical trials.
